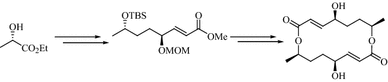
Stereoselective total synthesis of (−)-pyrenophorolDongamanti Ashok, Sridhar Pervaram, Venkata Ramana Reddy Chittireddy, Sreenivasulu Reddymasu, and Naresh Kumar Vuppula Osmania University, Hyderabad, India
E-mail: pervaramsridhar@gmail.com Abstract: A simple and efficient stereoselective synthesis of macrodilactone of (−)-pyrenophorol (1) has been accomplished in 12 steps in 8.3% overall yield, from inexpensive and commercially available (S)-ethyl lactate. This convergent synthesis utilizes an oxidation–reduction protocol and cyclodimerisation under the Mitsunobu reaction conditions as key steps.
Keywords: (S)-ethyl lactate ; Wittig olefination ; Intermolecular Mitsunobu cyclization ; (−)-Pyrenophorol. Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-017-0331-4
Chemical Papers 72 (4) 971–977 (2018) |
Wednesday, April 17, 2024 |
|||
© 2024 Chemical Papers |
||||